Step-Growth Polymerization
A step-growth polymerization is a stepwise reaction between bi-functional or mult-ifunctional monomers in which high-molecular-weight polymers are formed after a large number of steps. In contrat to chain-growth, all monomers are reactive. As a consequence, most monomers are consumed early in the polymerization to form short chains (oligomers) that combine to long polymer chains at a later stage of the polymerization.
Many naturally and synthetic polymers are produced by step-growth polymerization including polyesters, polyethers, urethanes, epoxies, and polyamides (see table below). Two well-known examples are the reaction of dicarboxylic acids with diamines to form polyamides (Nylon) and the reaction of organic diacids with alcohols to form polyesters, like polyethylene terephthalate (PET).
HOOC-R'-COOH + HO-R''-OH → HOOC-R'-COO-R''-OH + H2O
HOOC-R'-COOH + H2N-R''-NH2 → HOOC-R'-CONH-R''-NH2 + H2O
Due to the nature of the polymerization mechanism, the reaction has to proceed for a long time to achieve high molecular weight polymers. The easiest way to visualize a step-growth polymerization mechanism is a crowd of people reaching out to hold their hands to form human chains - each person has two hands (= two reactive sites) and can hold hands (form bonds) with two other persons.
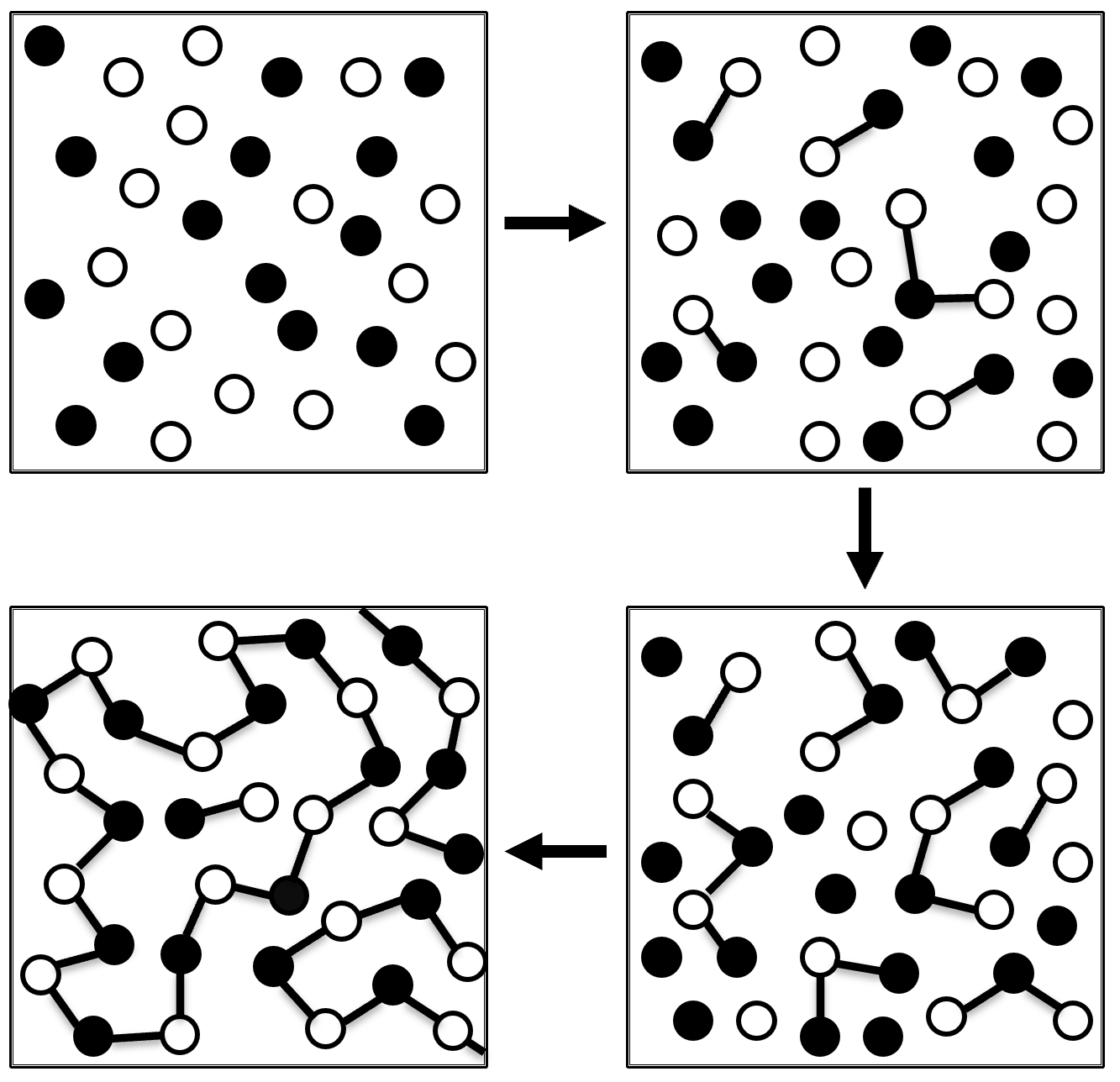
If some of the monomers have more than two reactive sites, branched or cross-linked polymers (thermosets) are formed.
Step-growth polymerization offers several advantages over chain growth polymerization; for example, no initiator is required to start the polymerization and termination reactions are absent. On the downside, long reaction times are typically required to achieve a high degrees of conversion and high molecular weights.
Some step-growth polymerizations of commercial importance are shown below.
| Functional Groups | Linkage | Polymer Type |
| -OH + -COOH | -C(=O)-O- | Polyester |
| -OH + -NCO | -O-C(=O)-NH- | Polyurethane |
| -NH2 + -NCO | -NH-C(=O)-NH- | Polyurea |
| -NH2 + -COOH | -NH-C(=O)- | Polyamide |
| -OH + -OH | -O- | Polyether |
Step-growth polymerizations can be divided into two lasses: condensation and addition polymerization.1 In the case of a condensation reaction, two monomers combine with the loss of a small molecule, usually an alcohol, a water or an acid molecule, whereas an addition reaction involves only the rearrangement of the electrons of a double bond to form a single bond with another molecule.
Notes
The terms addition and chain-growth are often used interchangeable but they are not synonyms. Chain polymerization is a polymerization mechanism in which monomer molecules add onto an active site of a growing polymer chain one at a time (IUPAC). Addition polymerization, on the other hand, is a polymerization in which the growth of polymer chains proceeds by addition reactions between molecules of any size (IUPAC). Earlier definitions of addition polymerization also included chain polymerization, but did not include condensation polymerization. Thus, addition, condensation, and step-growth are not synonyms as are not the terms addition and chain growth.
Revised July 23, 2020